 Tae Sik Sung, Jae Pyo Jeon, Byung Joo Kim, Chansik Hong, Sung Young Kim, Jinsung Kim, Ju Hong Jeon, Hyun Jin Kim, Chang Kook Suh, Seon Jeong Kim, Insuk So
Tae Sik Sung, Jae Pyo Jeon, Byung Joo Kim, Chansik Hong, Sung Young Kim, Jinsung Kim, Ju Hong Jeon, Hyun Jin Kim, Chang Kook Suh, Seon Jeong Kim, Insuk So
1. Department of Physiology and Institute of Dermatological Science, Seoul National University College of Medicine, Seoul;
2. Division of Longevity and Biofunctional Medicine, Pusan National University School of Korean Medicine, Yangsan;
3. Department of Physiology, Sungkyunkwan University School of Medicine, Suwon;
4. Department of Physiologyand Biophysics, Inha University College of Medicine, Incheon;
5. Center for Bio-Articial Muscle and Department of Biomedical Engineering, Hanyang University, Seoul, Republic of Korea
*Corresponding author.E-mail: insuk@plaza.snu.ac.kr.
원문 링크 : http://ajpcell.physiology.org/content/301/4/C823.long
Abstract
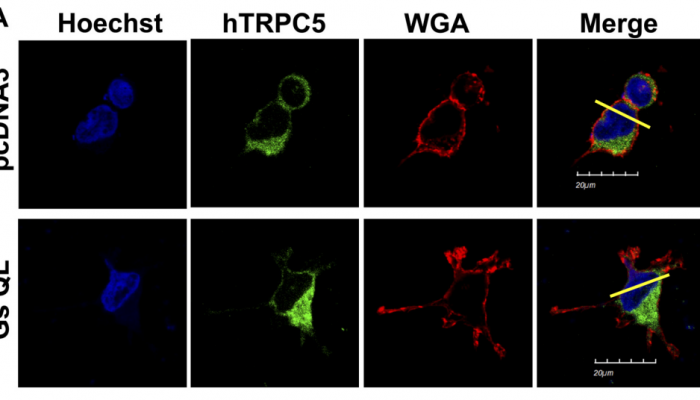
Canonical transient receptor potential (TRPC) channels are Ca2+-permeable, nonselective cation channels that are widely expressed in numerous cell types. Here, we demonstrate a new mechanism of TPRC isofom 5 (TRPC5) regulation, via cAMP signaling via Gαs. Monovalent cation currents in human embryonic kidney-293 cells transfected with TRPC5 were induced by G protein activation with intracellular perfusion of GTPγS or by muscarinic stimulation. This current could be inhibited by a membrane-permeable analog of cAMP, 8-bromo-cAMP, by isoproterenol, by a constitutively active form of Gαs [Gαs (Q227L)], and by forskolin. These inhibitory effects were blocked by the protein kinase A (PKA) inhibitors, KT-5720 and H-89, as well as by two point mutations at consensus PKA phosphorylation sites on TRPC5 (S794A and S796A). Surface expression of several mutated versions of TRPC5, quantified using surface biotinylation, were not affected by Gαs (Q227L), suggesting that trafficking of this channel does not underlie the regulation we report. This mechanism of inhibition was also found to be important for the closely related channel, TRPC4, in particular for TRPC4α, although TRPC4β was also affected. However, this form of regulation was not found to be involved in TRPC6 and transient receptor potential vanilloid 6 function. In murine intestinal smooth muscle cells, muscarinic stimulation-induced cation currents were mediated by TRPC4 (>80%) and TRPC6. In murine intestinal smooth muscle cells, 8-bromo-cAMP, adrenaline, and isoproterenol decreased nonselective cation currents activated by muscarinic stimulation or GTPγS. Together, these results suggest that TRPC5 is directly phosphorylated by Gs/cAMP/PKA at positions S794 and S796. This mechanism may be physiologically important in visceral tissues, where muscarinic receptor and β2-adrenergic receptor are involved in the relaxation and contraction of smooth muscles.